PROTOCOL AMENDMENT
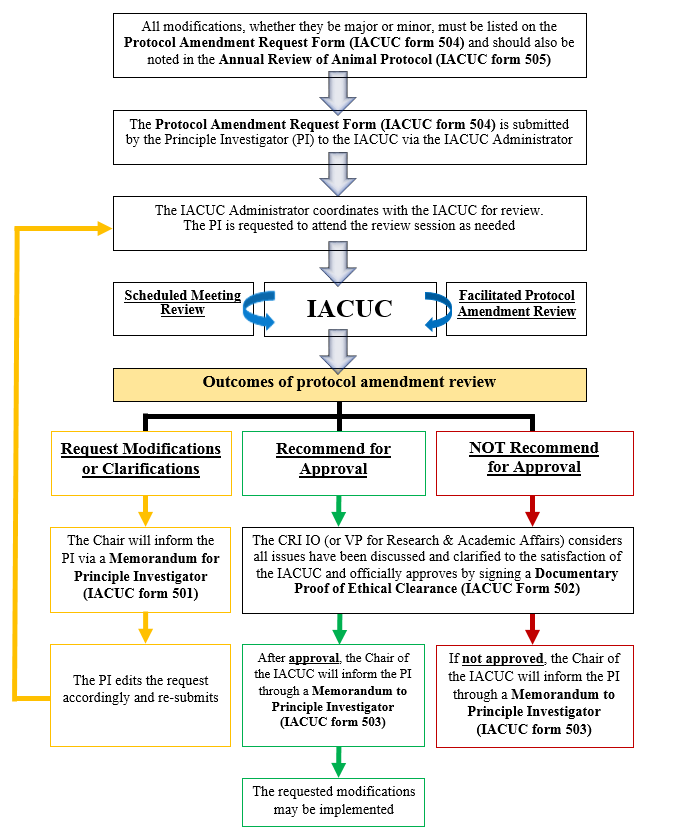
Proposed modifications to an approved protocol must be reviewed by the IACUC, and receive final approval from the IO (or Vice-president for Research and Academic Affairs in the case that the IO is part of the research team on the protocol) prior to start of the requested study. Modifications to animal protocols may be classified as minor or major. Minor modifications may include changing doses or routes of administration of a drug, and recording or measuring additional variables in the whole animals that are no more invasive than procedures currently approved under the protocol, and that do not increase the pain category of the protocol. Major modifications are those that require a change in the scientific direction of a protocol, e.g. requiring a greater than 10% increase in the number of test animals, changes in the species of the animals approved on the original protocol, changes in pain severity rating, increases in biohazard level on the protocol, and the addition of multiple minor modifications, or a change in the PI. A new PI for a previously approved and active protocol must submit a training statement with his/her request and a signed assurance statement. The process for reviewing/approving protocol amendments is the same as for reviewing a new protocol.
Link
1. LAU-IACUC Form 500 PROTOCOL COVER SHEET
2. LAU-IACUC Form 501 IACUC memorandum to PI (protocol review)
3. LAU-IACUC Form 502 Documentary Proof of Ethical Clearance
4. LAU-IACUC Form 503 IACUC memorandum to PI (Notification of Protocol Approval)
5. LAU-IACUC Form 504 Protocol Amendment
6. LAU-IACUC Form 505 Annual Review of Animal Protocol


