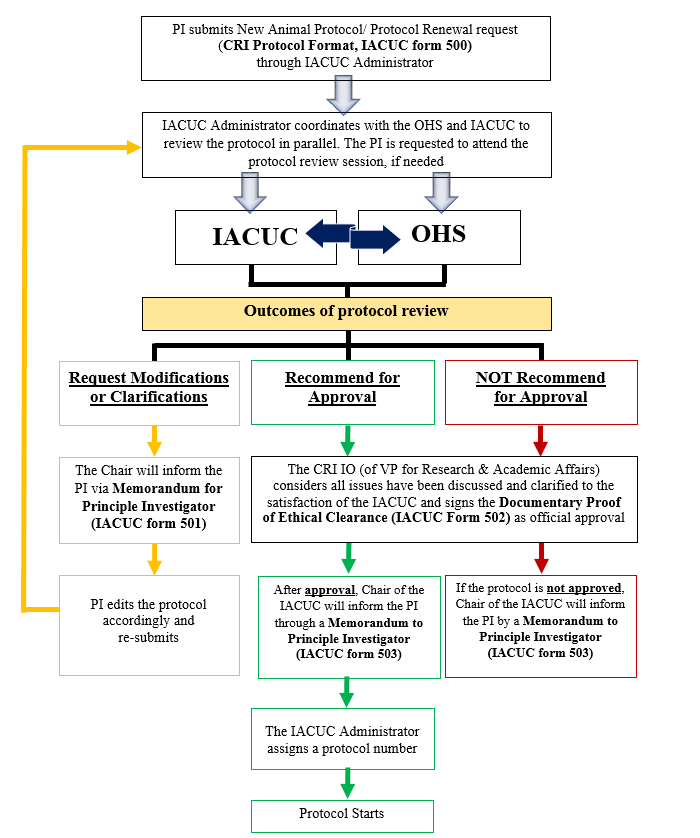
PROTOCOL REVIEW
The IACUC must review all proposed animal use protocols before any animal research can be performed within the CRI research and animal facilities. In reviewing the protocol, the IACUC will consider whether the protocol contains all required management and regulatory elements as defined by the Animal Use Policy and Laboratory Animal Program Description. The IACUC will determine if the proposal contains the following information: a justification of why the treatment/condition is scientifically necessary; a statement demonstrating that the PI performed a thorough search of alternatives to the potentially painful procedures; and standards and practices for pain management, adjuvant use, humane end-point determination, etc. At the same time, the OHS Committee will review and discuss safety matters involving the use of chemical, biohazardous and/or radiological agents or materials in animal protocols to ensure the investigator has appropriately planned to reduce or eliminate the exposure to potentially hazardous agents. After determination by the IACUC that the animal use protocol is complete, the protocol is recommended to the IO (or Vice-President for Research and Academic Affairs in the case that the IO is part of the research team on the protocol) for final approval. After official approval, the IACUC Chair will inform the PI that the study may begin.
Link
1. LAU-IACUC Form 500 PROTOCOL COVER SHEET
2. LAU-IACUC Form 501 IACUC memorandum to PI (protocol review)
3. LAU-IACUC Form 502 Documentary Proof of Ethical Clearance
4. LAU-IACUC Form 503 IACUC memorandum to PI (Notification of Protocol Approval)


