Stress responses in pathogenic bacteria

Stress responses in pathogenic bacteria
The Laboratory of Biotechnology is also interested in genes that contribute to bacterial pathogenesis. During infection, bacteria have to evade and defend against host immune responses. White blood cells, such as macrophages and neutrophils, are the key players in the host innate immune response to infection (Fig. A). These cells generate reactive oxygen and nitrogen species in order to kill invading bacteria. The Laboratory is focused on the study of the genes and enzymes that bacteria use to protect themselves from reactive oxygen species. A more complete understanding of these bacterial defense mechanisms will contribute to one of the laboratory’s main goals, which is to gain insights that can lead to the development of more effective treatments for bacterial infections.
One active area of research involves the study of how t-RNA modifications by enzymes such as the methylase, TrmB, increase the translation rate of mRNAs encoding oxidative stress defense proteins such as catalases (Fig. C). The proteins involved in these processes, such as TrmB, are not found in humans and are therefore attractive targets for the development of novel antibacterial therapies. Other lines of enquiry are probing the relationships between oxidative stress and the expression of xenobiotic and antibiotic efflux pumps. Studies of the effect of environmental stresses on bacterial pathogenicity and resistance to antimicrobial compounds are also ongoing.
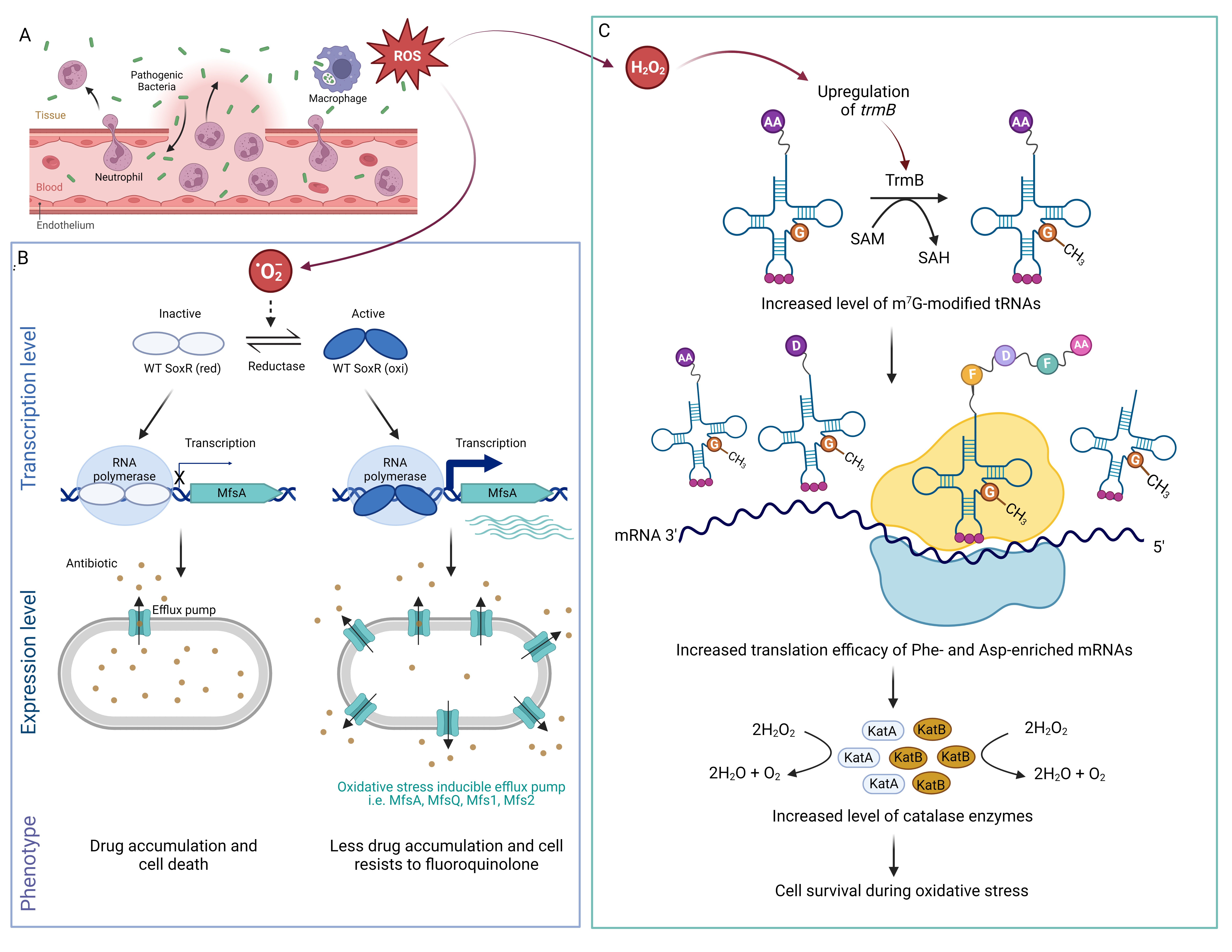
Figure. A schematic of the host response to bacterial infection showing the production of ROS by immune cells (A); the bacterial response to oxidative stress during host-microbe interaction in which superoxide anion (·O2–) activates SoxR leading to increased mfsA expression levels resulting in a fluoroquinolone resistance phenotype (B). Hydrogen peroxide (H2O2) up-regulates TrmB production, thus increasing the level of m7G-modified tRNA, which enhances translation of Phe and Asp enriched H2O2 detoxifying enzymes resulting in cell survival during oxidative stress (C).

