Cancer Biomarkers
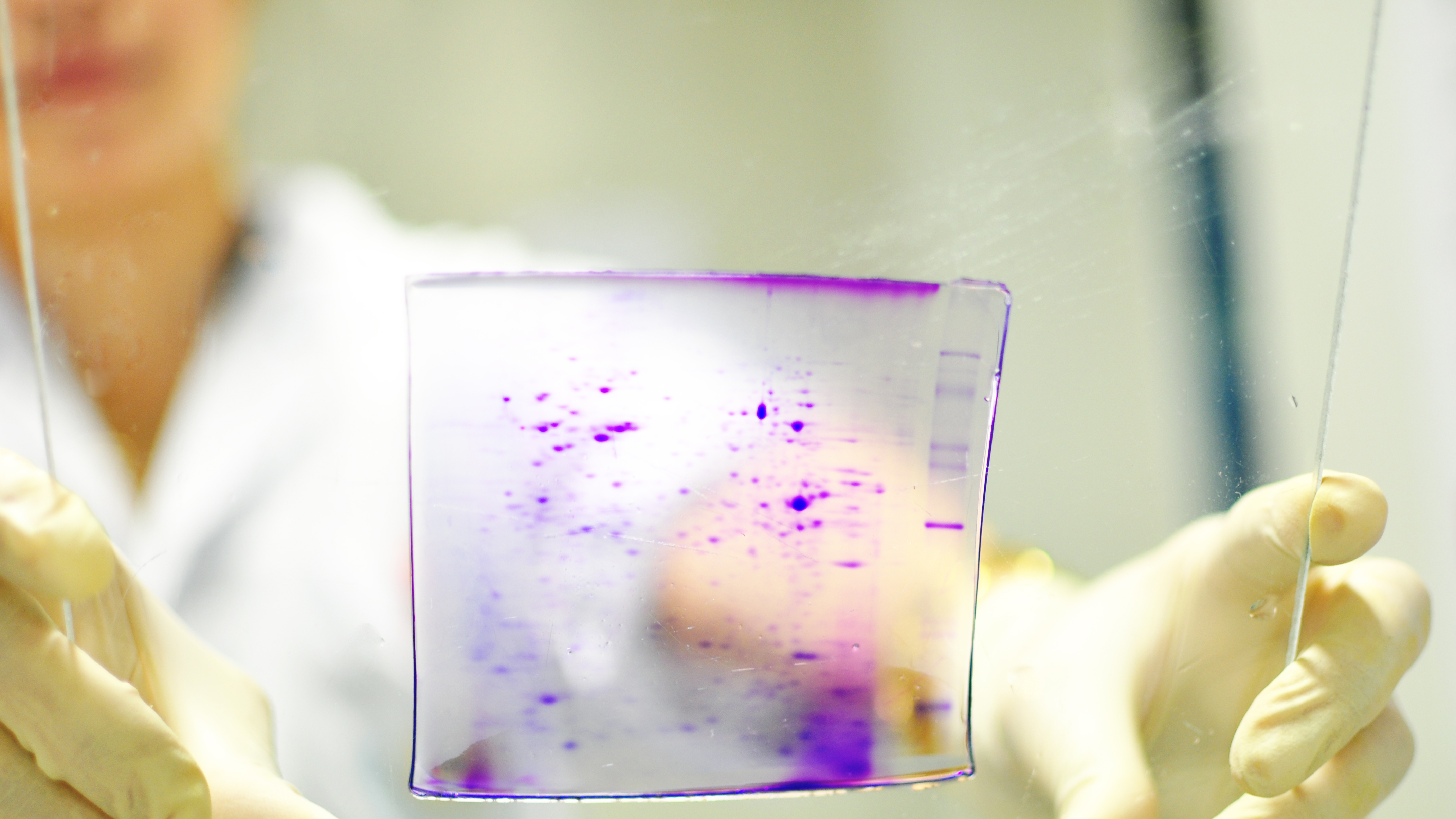
Cancer is initiated by accumulated mutations and proliferation to form a tumor. Often cancers may be cured if diagnosis and treatment can be made at the early stages, but there are still few biomarkers that will distinguish cancer tissues from normal tissues or that are specific for a particular cancer. Our research group was the first in Thailand to use the technique of proteomics, where the proteome patterns (or total protein present at any tissue under any given condition) are compared, such as by two-dimensional electrophoresis. Initial studies, in collaboration with Phramongkutklao Hospital, showed that increased expression of proteins, cathepsin B and prohibitin, was observed in neoplastic thyroid diseases compared to non-neoplastic diseases, such as goiter or nodular hypoplasia, while high levels of expression of galectin-3 appeared to correlate with metastatic potential.
Since liver cancer is prevalent in North Thailand and bile duct cancer is prevalent in Northeast Thailand, extensive proteomic studies have been performed on hepatocellular carcinoma (HCC-S102, HepG2) and cholangiocarcinoma (HuCCA-1) cell lines. Comparison of proteins from these cell lines, suggest various potential biomarkers for distinguishing the two cancer types. Furthermore, study using patient tissues confirmed that the protein lipocalin 2 was detectable in all cancer tissues, but not in the normal tissues of cholangiocarcinoma patients, indicating its potential as a biomarker. Further studies on hepatocellular and cholangiocarcinoma cells focus on using three-dimensional culture techniques to mimic natural tumour growth and microenvironment, as well as studying subcellular fractions, such as secretomes, and improving culture conditions to increase protein yield for proteomics.


Another interest is the role of post-translational modification in cancer, particularly O-GlcNAc (O-linked N-acetylglucosamine), a single sugar molecule attachment occurring mainly on serine and threonine residues of cytoplasmic and nuclear proteins. This GlcNAc modification may act as a nutrient sensor through the hexosamine biosynthesis pathway, a minor branch of glycolysis, and may also affect cellular regulation, since it occurs on amino acids that may be phosphorylated. Study of O-GlcNAc modified proteins in breast and colon cancer tissues compared to adjacent normal tissues indicate increased GlcNAcylation in both cancers and indicated possible biomarkers. RNA interference of OGT, an enzyme catalyzing O-GlcNAcylation, decreased global O-GlcNAc levels and reduced colony formation of breast (MDA-MB-231 and MCF-7) and colon (HT29, SW480, and SW620) cancer cells, thus suggesting that O-GlcNAcylation plays important roles in cancer progression. Other studies on breast and colon cancer cells showed more GlcNAc modified proteins in the extracellular compartments of breast and colon cancer cells compared to their normal cells.
Our current interest has expanded to explore abnormality of extracellular glycoproteins from serum/plasma of cancer patients. Proteins in blood circulation system may contain some important biomolecules which reflect the pathological and physiological states of diseases including cancer. Aberrant O-linked and N-linked glycosylation of serum proteins has been examined in many types of cancer. Indeed, research on glycoproteomics, glycosylation patterns and site specific glycans on proteins are being applied to discover the heterogeneity of glycan structures on glycoproteins. Thus, we recently found that several glycoforms of serum glycoproteins were altered in patients with colorectal cancer. This indicates that abnormality of glycoforms on glycoproteins may provide a novel frontier for cancer biomarker discovery

